Patient safety seeks a boost from improved practices in anatomic and digital pathology
By Steve Halasey
Anatomic pathology is acknowledged to be a demanding field in which personal experience and professional technique often play important roles in the success of daily practice. But such reliance on the skill of individual practitioners is just as often viewed as a weakness of the field, making it dependent on unstandardized and manual processes that are subject to error and place patient safety at risk.
Earlier this year, an international panel of leaders in the pathology community sought to initiate a change in this status quo by endorsing a statement of principles intended to drive positive change and improve patient safety in the anatomic pathology laboratory. Convened as the Ventana International Pathology Patient Safety Advisory Board at the invitation of Ventana Medical Systems Inc, Tucson, Ariz—a member of the Roche Group—the panel adopted a set of “Principles for Pathology and Patient Safety” emphasizing the need for standardization in pathology practices.1,2
[reference id=”39489″]Sidebar: Ventana International Pathology Patient Safety Advisory Board[/reference]
The efforts of the panel did not end with the adoption of its patient safety principles. “Since February, we’ve devoted time to discussing activities that will be conducted under a broader Patient Safety Initiative, such as understanding the different patient safety performance and quality metrics that are currently used by individual institutions, and developing recommendations for standardized metrics,” says Dr Eslie Dennis, MBChB, vice president of scientific affairs for Ventana and chair of the company’s advisory panel.
John Carpenter, MD, a pathologist at Sound Pathology Associates LLC, Lynnwood, Wash, was invited to serve as a member of the advisory board and to share his perspective on patient safety issues in anatomic pathology. “I hope that the initiative will help define issues related to patient safety in the histology laboratory, generate discussion in the pathology community regarding ways to improve our practices, and perhaps prompt further study of methods or technologies that could mitigate some of the inherent risks and limitations in anatomic pathology.”
“We are also developing proposals to identify gaps and define the extent of patient safety issues. Our recent efforts have been focused on gathering information and understanding the patient safety landscape in greater detail,” says Dennis.
Since compiling its statement of principles, Ventana’s advisory panel has also sought to carry its message to other organizations with an interest in the field. “To increase awareness of patient safety risks in the lab, we’ve shared the principles at conferences hosted by the United States & Canadian Academy of Pathology, the National Patient Safety Foundation, the National Society of Histotechnology, and the European Congress of Pathology. In October, we will be at meetings of the American Society for Healthcare Risk Management and the International Academy of Pathology,” says Dennis.
Other organizations are also following the topics identified by the Ventana panel. “The College of American Pathologists laboratory accreditation program is very focused on patient safety issues, some of which are also addressed by the Ventana initiative,” says Carpenter.
“We are very encouraged by responses we’ve received to the Patient Safety Principles, because they signal a real desire to improve operational safety, quality, and efficiency in the anatomic pathology laboratory,” says Dennis. “Still, significant challenges remain.”
For this article, CLP contacted a number of experts in the field—including members of the Ventana panel—to better understand the patient safety issues now of concern to the pathology community.
RESPONDING TO CHALLENGES
In its initial discussions and statement of principles, Ventana’s advisory board noted that there are opportunities to enhance patient safety by developing solutions to improve tissue preservation, specimen identification and tracking, and prevention of tissue contamination. According to Dennis, the panel’s call has hit a responsive note among professionals in the field.
Tissue Preservation
Preanalytic variables begin to affect tissues virtually from the moment a patient undergoes a procedure and specimens are obtained.
“To reduce as many preanalytical variables as possible, we need a consistent quality for all tissue specimens that enter the analytical workflow in the lab,” says Ralf Huss, MD, chief medical officer at Definiens, based in Munich, Germany, a company that offers image analysis solutions for tissue diagnostics. “This should include documented warm and cold ischemia time, defined fixation procedures (preferably with the same method and fixative), in-process controls that document any process deviations, validated processes and devices, and so on.”
“Differences in local practices regarding specimen handling, labeling, preservation, and transport result in variability of tissue quality,” agrees Dennis. “Although robust technology offerings may deliver the increased benefits of safety, quality, and efficiency, issues that arise before the specimen arrives at the lab remain beyond the lab’s control. As pathologists have no control over specimen-related activities outside the lab, standardization throughout the specimen chain of custody is another challenge. This situation highlights the need for laboratory and hospital administrators to be more involved with the many practices related to specimen submission to the lab.”
Huss adds that the same level of rigor should also be applied to reducing variation for analytical variables. “We need standardization of staining and molecular procedures with appropriate in-process controls,” he says. “Digital quantitative pathology is a pivotal tool to monitor, control, and document most of the analytical steps.”
Specimen Identification and Tracking
Another issue that arises during the preanalytical phase is specimen tissue identification. Addressing the issue at this stage—and maintaining identification throughout the tissue’s lab journey—represents another significant quality assurance mission for practicing anatomic pathologists.
Guarding against interpretive errors is a significant and valued role of trained and experienced subspecialist pathologists, say the experts. But disastrous results can occur if these skills are directed toward slides that have been labeled with the incorrect patient or test information. Similarly, pathologist efficiency and timely performance of additional studies can suffer if there are delays in slide delivery or unclear communications between the pathologist and laboratory.
“Over the past 7 years, we have implemented barcode-driven processes that greatly reduce, but never completely eliminate, these risks,” says Pat Cooke, chief information officer at CellNetix Pathology & Laboratories, a private pathology company with offices in Washington State and Alaska. “We track the specimen from pick-up at a client site all the way to arrival at the pathologist’s desk. ‘Just-in-time’ labeling at microtomy has been one of our most successful areas of error reduction in terms of misidentification and missing specimens.”

Barcoded patient specimen is placed in automated slide staining system. (Names are fictitious.) Photo courtesy Ventana.
“Barcode-based tracking systems implemented with carefully devised workflow systems have been shown to significantly reduce identification errors,” says Dennis. “Automation reduces the number of manual touch points and consequently can help reduce the number of errors that can occur across the lab work stream.”
As the volume of testing in anatomic pathology labs increases, each human interaction, while necessary, also provides an opportunity for error. Printing labels at the time they are actually used (instead of the traditional “batching” workflow), and using barcode scanning to track specimens through the stations of grossing, microtomy, staining, and slide delivery provides for improved specimen integrity.
“For the pathologist, an additional benefit of barcoded specimens is improved case visibility—down to the status of any individual specimen as it moves throughout the lab. This can be very helpful in providing accurate feedback to clinicians and ordering additional stains or molecular studies quicker,” says Matthew P. Horton, MD, medical director at CellNetix. “And should a problem arise, one can analyze the embedding logs, cutting logs, and so forth to pinpoint the root cause of the error or contamination. Such tracking can facilitate procedural reviews and additional training as necessary to prevent the error from recurring.”
On the high end, sophisticated use of integrated specimen tracking systems can lead to another opportunity for improvements by way of upstream connectivity to information systems outside the pathology lab. “Today’s pathology laboratories should be involved with the standardization of terms and data language to ensure the universal flow of information,” says Dennis. “Fragmented systems introduce another safety risk in transferring information from one system to another. Lab and hospital administrators who recognize the value of increased automation and connectivity are increasingly investing in these strategic data solutions, demonstrating their commitment to improving patient safety in the anatomic pathology lab.”
Prevention of Tissue Contamination
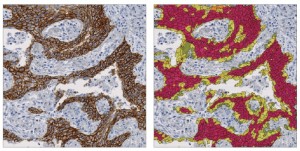
Quantitative digital pathology imaging can consistently quantify biomarker and target expression in a broad range of tissues, as in this example from Definiens.
The large number of manual touch points in the anatomic pathology lab sequence—where each touch point increases the risk of contamination—remains among the most serious of challenges. One reason contamination is such a difficult problem is that contaminants can be introduced (and not easily recognized) in any one of the multiple laboratory stages, from grossing and processing through embedding and staining.
“Of all the technical obstacles inherent in the histology laboratory, tissue contamination may be the most difficult to address,” says Sound Pathology’s Carpenter. “The preparation of slides requires many detailed, manual processes that are subject to human error, and utilizes many steps with batch processing in liquid media. Contaminants can be introduced at any point in the life cycle of a pathology specimen, including at the bedside, grossing, tissue processing, embedding, cutting, and staining. Furthermore, there are numerous mechanisms that can lead to tissue contamination at each of those steps.”
In contrast to the problem of misidentification, where the application of technologies can resolve many issues, contamination is a tougher nut to crack. “It is more difficult to find the root cause of cross-contamination with technology,” says CellNetix’s Cooke.
“Thus, strong procedures and effective training are highly important,” adds Janice Caputo, anatomic pathology product manager for Sunquest Information Systems, Tucson, Ariz, a leading provider of software solutions for managing laboratory and diagnostic processes.
“Tissue contamination will probably always be an obstacle,” says Carpenter, “but our challenge is to identify methods and technologies that can mitigate the risk.
“Despite the technical challenges involved,” he adds, “it should be noted that clinically significant events related to unrecognized tissue contamination are relatively rare.”
“However, we believe there is significant under-reporting,” says Dennis, “so we don’t really know what we don’t know. Fortunately, there are technologies available that mitigate staining process contamination by taking an individual specimen staining approach, as opposed to the traditional batch staining approach.”
PROFESSIONAL EDUCATION

Ventana’s Vantage workflow tracking station for microtomy images and data. (Names are fictitious.) Photo courtesy Ventana.
Although the application of advanced technologies will play a key role in resolving many of the challenges afflicting the field of anatomic pathology, the experts agree that technology alone is not the answer.
“While many challenges exist, improvements can be made with increased education, awareness, monitoring, and ensuring lab personnel are following proper tissue sample processing and staining protocols,” says Dennis.
“In my view,” agrees Carpenter, “the primary challenge that pathologists (or any physicians) face in delivering the safest care possible is maintaining excellent diagnostic skills and thorough knowledge of the clinical situation of each individual patient.”
“A key opportunity for improvement of lab quality centers on the implementation of standard operating procedures for preanalytical and analytical processes, coupled with a ‘quality-by-design’ approach to achieving appropriate quality measures through the use of certified devices operated by qualified personnel,” says Huss.
“The initial investment required to launch such an effort is feared by many laboratory administrators and their financial departments. But a long-term return on investment will be realized through improved and consistent test quality, and an increasing number of tests ordered by a growing number of satisfied customers,” says Huss. “In the future, this will be the only way to certify standard pathology lab quality, and digital pathology will be a pivotal part of it.”
A first step toward achieving standardization in anatomic pathology labs could be to define and implement a common set of patient safety and quality metrics that all labs can use to track and measure their performance.
The Vantage tracking station uses touchscreen technology. (Names are fictitious.) Photo courtesy Ventana.
“I would like to see more data that describes the frequency and severity of contamination and labeling errors in pathology practices, as well as how much improvement is actually observed following implementation of various mitigation techniques,” says Carpenter. “We have some idea of the scope of the problem from small studies and case reports, but we don’t have a complete understanding of the current extent or clinical significance of contamination and mislabeling events.”
“Currently, there is no common set of metrics used on a routine basis,” agrees Dennis. “Through the Patient Safety Initiative, we’ll be developing recommended patient safety quality metrics. And to help labs universally adopt these recommendations, we’ll provide the metric rationale and define a new vision of how the patient safety landscape could ideally look.”
“We would like the guideline bodies to consider incorporating these recommendations to encourage standardized implementation, and subsequently to encourage the anonymous collection of these metrics to allow for industry benchmarking,” adds Dennis.
In the absence of such benchmarking data to define the scope and severity of quality and patient safety issues affecting pathology labs, experts hold varying views about whether stronger guidance materials and professional practice protocols are needed to improve lab performance.
“There is a need to improve and standardize the performance of pathology procedures, so that labs can reach results with the least possible interobserver and interlab variability,” says Huss. “This is currently done by interlaboratory performance studies—so-called ‘ring trials’—mostly on a trial-and-error basis instead of using a systematic approach. The development of quantitative digital pathology will provide a systematic solution for this need, as it can consistently quantify biomarker and target expression in a broad range of tissues.”
Sound Pathology’s Carpenter is less certain that lab performance data have so far demonstrated a need to make significant revisions to established guidelines. “Most laboratories operate under the relatively standardized guidance of the College of American Pathologists guidelines and protocols,” says Carpenter. “At this point, I am not sure that additional protocols are needed or justified.”
Moreover, adds Carpenter, even the adoption of new laboratory technologies should be subjected to scrutiny in the light of stronger data about the issues. “We assume that better technology will help mitigate risk, and this is probably true,” says Carpenter, “but strong data is always needed to support changes in practice standards.”
Nevertheless, some technologies already have a track record that may move them to the head of the queue for laboratory implementation. “The barcoding of specimens is a proven solution in other areas of patient care, and it should be also accepted as the new standard of care for tracking specimens in anatomic pathology—from the point of collection through diagnosis,” says CellNetix’s Cooke. “We support a mandatory requirement for barcoded specimen tracking in the anatomic pathology lab, and believe that the College of American Pathologists and the Joint Commission would be the appropriate bodies to enforce this.”
TECHNOLOGY ADVANCES
Because of its reliance on difficult-to-automate manual processes, the field of anatomic pathology has rarely been considered amenable to improvement through the implementation of new technologies. With continued advances in the fields of automation, molecular biology, image processing, and information technologies, however, old views are changing.
In today’s environment, experts are less skeptical that technology will eventually address all of the major challenges that arise in pathology labs, and more likely to support the continued development of promising new systems. But for the time being, the list of problems to be solved still outnumbers the available solutions.
Technologies for standardizing tissue fixation and processing—the most labor-intensive stages of anatomic pathology—are high on the list of laboratory needs. “To address the problems of preanalytic variability, new technologies for tissue preservation are needed to stabilize, preserve, and maintain proteins, nucleic acids, and tissue architecture,” says Dennis.

Screenshot of the Vantage tracking station for microtomy images and data. (Names are fictitious.) Photo courtesy Ventana.
Preventing errors that can arise through the misidentification of patient specimens is also a top goal of pathologists. “A gap in the chain of custody exists between the patient and the lab, beginning at specimen collection,” says CellNetix’s Horton. “Hospitals are already improving positive patient identification with tools such as double labeling or barcoded wristbands in the phlebotomy and clinical pathology space. As this focus extends to operating rooms and endoscopy suites, anatomic pathology labs will be mandated to employ similar positive patient identification techniques. Barcoding specimens at the time of receipt permits specimen tracking throughout the course of testing, concluding only when the pathologist signs out the case.”
CellNetix is partnering with Sunquest to pursue advances in software-based positive material identification. “There is also untapped potential in radio-frequency identification tags,” says Cooke. “But for now, we feel that widespread adoption of barcode-driven specimen tracking methodologies should be the priority for anatomic pathology.”
The emergence of digital pathology is also expected to bring about significant changes in the technology component of pathology labs, with potentially significant benefits for patient safety. “Most of the necessary technologies and platforms exist, but they don’t ‘speak’ to each other—even if they are validated and qualified,” says Huss. “Most analyses are done in an isolated manner, but the results need to be put together into a common clinical report. Digital pathology is one way to generate such comprehensive reports that also documents the quality of the analyses performed, and informs the clinician (and the patient) how a diagnosis was reached.”
CONCLUSION
According to Dennis, two factors will ultimately be key to the success of the patient safety initiative. “First, collaboration among all stakeholders (including hospital administrators) involved with patients and their specimens. And second, universal adoption of standardized processes and metrics for tissue processing, quality, and patient safety.”
How rapidly the professional community is prepared to make commitments to these goals remains to be seen. But with initiatives under way from a variety of sources, improved procedures for anatomic pathology seem to be on the horizon.
Steve Halasey is chief editor of CLP. He can be reached via [email protected].
REFERENCES
1. Inside track: pathology and patient safety. Clinical Lab Products. 2104;44(4):34; expanded version online at www.clpmag.com/2014/03/inside-track-pathology-patient-safety.
2. International panel of pathology leaders develop principles for patient safety. CLP Prime. 2014; Epub March 13, 2014; available at: www.clpmag.com/2014/03/international-panel-pathology-leaders-develop-principles-patient-safety.
Top photo caption: Barcoding pathology specimens at the time of receipt permits specimen tracking throughout the course of testing. (Names are fictitious.) Photo courtesy Ventana.