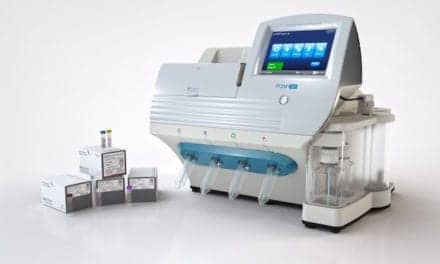
In vitro diagnostics manufacturer Beckman Coulter Diagnostics, Brea, Calif, has recently entered into a supply agreement with Seegene Inc, Seoul, Republic of Korea, a developer of multiplex PCR technologies. Under the agreement, Seegene will manufacture reagents designed exclusively for Beckman Coulter’s new sample-to-answer molecular diagnostics instrument, the Veris molecular diagnostics system.
“We’re very excited to partner with Seegene and incorporate their novel and proprietary dual priming oligonucleotide (DPO), tagging oligonucleotide capture and extension (TOCE), and multiple detection temperature (MuDT) technologies into our MDx solution,” says Richard Creager, senior vice president of the molecular diagnostics business unit and chief scientific officer at Beckman Coulter. “We expect to be able to quickly add to the menu we offer on Veris with the Seegene multiplex reagents that enable simultaneous and multiple detection and identification of various types of pathogens in a single test.”
The Veris system and Veris human cytomegalovirus (CMV) assay have received the CE mark, permitting market entry in Europe. The system is still in development for the United States, and is not yet available for in vitro diagnostic use in US markets.
“Our observation is that the Veris system breaks new ground in enabling laboratories to perform MDx testing in the most efficient way possible,” says Seegene founder and CEO, Jyong-Yoon Chun, PhD. “This partnership fully supports our promise to contribute to humanity by developing faster, more accurate, and cost-effective innovative MDx systems.”
For further information, visit Beckman Coulter Diagnostics.