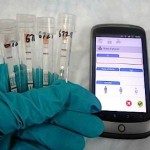
Corgenix and Fio are integrating rapid testing and mobile data capture technology to help track the West African ebola outbreak in real time. Courtesy Fio Corp.
Corgenix Medical Corp, Denver, and Fio Corp, Toronto, Canada, have announced that they are working together to integrate two technological advances to help end the ebola outbreak in West Africa: a rapid test, backed by the Bill & Melinda Gates Foundation and the Paul G. Allen Family Foundation, that can detect the virus in minutes; and a mobile device, which is being adapted with further funding from the Gates foundation, to analyze and upload results directly to a central data system.
Fio is adapting its Deki reader to work with the Corgenix ReEboV antigen rapid test for the ebola virus, which recently received FDA emergency use authorization and has been listed as eligible for procurement by the World Health Organization (WHO). Combining these technologies will give suspected ebola patients in the most remote, resource-poor settings access to automated test results. Results will then immediately be transmitted to Fio’s data system, Fionet, capturing vital information that is currently missed or delayed.
“This collaboration makes testing more accessible to those at risk, and data more readily available to those managing the outbreak—a combination that the Gates foundation identified and mobilized,” says Michael M. Greenberg, MD, chairman and CEO of Fio. “The resulting technology will expand the capability to fight ebola now and strengthen national health systems beyond the current crisis.”
Corgenix researchers on the ground in Sierra Leone have already started using smartphones to transmit images of rapid ebola test results to the Fionet data system. Together with Fio’s tools for case and contact mapping, this capability offers governments and other organizations responding to the outbreak a way to track the disease in real time.
“We’re taking the fight against ebola to another level with Fio’s data collection and management, which is critical to the success of rapid testing,” says Douglass Simpson, Corgenix president and CEO. “This marks a tremendous advancement in ebola testing, and it lays the groundwork for a strong and productive partnership between our two companies on future projects.”
The instant traceability of infected or potentially infected individuals—knowing test results, locations, and movement over time and across borders—is essential to containing the spread of the ebola virus and other highly contagious diseases.
Corgenix develops and manufactures specialized diagnostic kits for immunology disorders, vascular diseases, bone and joint disorders, and a line of unique detection products for viral hemorrhagic disease. The company currently sells more than 50 diagnostic products through its global distribution network. At its FDA-compliant and ISO 13485:2012-certified facilities in Colorado, Corgenix also serves as a contract developer and manufacturer of products for key medical and life science companies.
Fio is focused on decentralized management of infectious diseases in developed and developing countries. The company helps health workers improve the quality of care they provide to individual patients, while automatically capturing and disseminating data to strengthen health systems as a whole. Fio’s solution combines mobile intelligent devices with cloud information services to facilitate healthcare delivery.
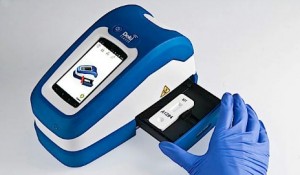
Fio’s Deki reader will help enable real-time ebola tracking by providing an automated analysis of rapid test results and immediately transmitting case reports to the Fionet cloud database. Courtesy Fio Corp.
Both companies will continue to work together to integrate the two technologies for decentralized ebola virus testing throughout West Africa. Beyond the current crisis, this will serve health systems more broadly to better manage future outbreaks, as well as other infectious diseases in the region, such as Lassa virus. Fio’s Deki reader—which is already CE-marked for use with rapid tests that detect malaria and dengue fever—is also being adapted for use with the Corgenix ReLasV antigen rapid test for the Lassa virus.
Fio received a Gates foundation grant in December 2014 to adapt its Deki reader to analyze rapid ebola tests and transmit results to Fionet to be integrated with case and contact management tools. A few weeks later, Corgenix received two grants—one from the Gates foundation and one from the Allen foundation—to advance the company’s development of an ebola rapid test kit.
The ReLasV and ReEboV antigen rapid tests have not been cleared by FDA for routine diagnostic use. The ReLasV test is CE marked for diagnostic use in the European Union and other countries that recognize the CE mark. The ReEboV test is not CE marked and may only be used as a diagnostic device under the conditions of FDA’s emergency use authorization.
For additional information, visit Corgenix Medical Corp, and Fio Corp.







