Cutting-edge R&D will reshape and broaden the future of lab testing
By Gary Tufel
Diagnostic tools are constantly evolving and advancing. Among the many new technologies that are in development or gaining approval and entering the market, the advances described here are examples of new offerings that are particularly noteworthy for laboratorians as well as for physicians and patients.
THE POCKET-SIZED LAB
A new and unique pocket-sized lab could provide doctors immediate test results, reduce costs for budget-strapped clinical laboratories, and serve as a lab-testing tool for doctors working in remote areas.
“Lab-on-a-chip” (LOC) technology is the result of nearly 2 decades of development. The latest advance is the result of turning loose computer engineers using computer-aided design to develop LOC designs.
The new chip is the brainchild of Michigan Technological University computer engineers Shiyan Hu, PhD, associate professor of electrical and computer engineering, and doctoral student Chen Liao, who created a computer-generated biochip layout for routing a droplet of blood or other body fluid through multiple LOC channels. Their invention would facilitate running dozens of different diagnostic tests, from HIV to diabetes, using a single chip.
“Microfluidic biochip technology has gained tremendous success in the past decade. Using such a technology, some cumbersome equipment in the biochemical laboratory can be miniaturized and integrated into a single chip, and traditional biomedical experiments can be conducted there,” says Hu. “But despite its success, this technology has two major limitations.”
First, he says, a blood sample can be used to measure only a few quantities. “Currently, only up to thousands of biochemical reactions can be integrated into a single biochip. This is inherently due to the fact that prevailing biochips are designed manually, and a small group of designers has only limited capability.
“But if a single biochip could simultaneously execute a few bioassays corresponding to related diseases, then the clinical diagnoses would be much cheaper and more convenient to perform. Our multitest medical lab-on-a-chip work basically addresses this issue.”
“Second,” says Hu, “biochip-based detection is still error-prone due to the ubiquitous randomness and high complexity associated with biochemical reactions.” In other words, the correctness of biochip-based detection is not guaranteed. In practice, the same experiments have to be performed multiple times in order to validate measurement results that are not convenient or economical.
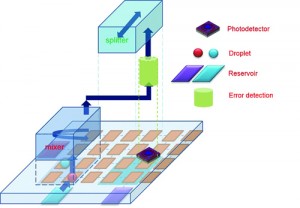
Automated design for on-chip detection integrated microfluidic biochips, by Shiyan Hu, PhD, Michigan Technological University.
“Our current research is seeking to address this issue through the deployment of on-chip sensors. Such on-chip sensors can closely monitor the intermediate results of some critical steps in the on-chip biochemical reactions. In turn, the correctness of those biochemical operations can be validated inside the biochip, and thus the whole experiment can be deemed trustworthy,” says Hu. “We are working on developing advanced algorithmic techniques for biochip and on-chip sensor codesign, such that repetitive experiments are no longer necessary and the waste of blood samples is significantly reduced.”
SMALL SAMPLE, EARLY RESULTS
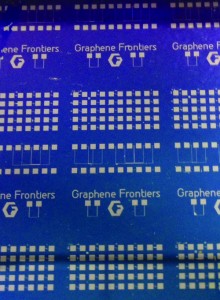
Graphene Frontiers, Philadelphia, began operations in 2010 and became a spinout under the University of Pennsylvania’s UPstart program in 2011. The company has developed a graphene-based diagnostic technology that promises to make routine testing more efficient by using an extremely small blood sample for early detection of a number of diseases and biomarkers, including high levels of troponin often associated with heart attacks.
According to Mike Patterson, CEO of Graphene Frontiers, the company’s noninvasive medical device technology will be suitable for use as a part of routine annual physical examinations, and has the ability to detect many diseases in their earliest stages using only a small blood sample. The technology is still about 5 to 10 years away from commercialization; a graphene field effect transistor (GFET) prototype is now under development.
Graphene Frontiers aims to be one of the first companies to successfully use the hypersensitive, conductive properties of graphene in medical devices. The company’s approach hasn’t been perfected yet, but the company has earned key patents and developed a unique roll-to-roll process for mass production.
The core technology for graphene production was developed in the physics department at the University of Pennsylvania. “To make graphene, we use atmospheric pressure chemical vapor deposition. Basically, we use heat and copper to turn the carbon in a gas-like methane into a single atomic layer ‘film’ of graphene,” says Patterson.
“In our lab in Philadelphia—and in partnership with our founder, Charlie Johnson, and the Nano/Bio Interface Center at Penn—we have developed a simple electronic device based on graphene that we can use to detect chemicals and biomolecules. These devices have a functional area of about 10 microns—that is, about one-tenth the width of a human hair. We can put hundreds of variations of functionalized sensors on a chip smaller than a postage stamp to perform massively multiplexed testing. We use a tiny electric current to detect when a target analyte, such as the bacteria in a blood sample, binds to the sensor channel. Using this simple, all-electronic, before-and-after measurement, we can detect and quantify the analyte of interest in near real-time.”
The technique improves testing and diagnoses for patients and for clinical labs, offering testing advantages for a variety of diseases. “The way lab testing works today is simply not good enough,” Patterson says.

A sheet of copper being fed into the kiln during the chemical vapor deposition phase of graphene production.
In place of the familiar and protracted processes currently required to perform clinical lab testing and get a diagnosis, Patterson offers a vision of the way it should work. A patient visits a primary care physician or urgent care clinic, where a medical professional (doctor, physician’s assistant, or nurse) asks several appropriate screening questions. The medical professional then takes a small sample (like a drop of blood from a fingerstick), and uses a graphene-based microfluidic cartridge and reader to test for dozens of targets. Within minutes, the medical professional is able to review the results with the patient. Patterson lists the advantages of the new technology:
- Very sensitive (permits early detection of targets in low concentrations);
- Very selective (reducing false positives);
- Ability to quantify (monitoring levels over time);
- Ability to massively multiplex (potentially testing for hundreds of analytes in a single sample);
- Fast (first result in minutes, potentially saving lives);
- All electronic (no expensive or sensitive optical or other readers);
- Simple (no need for trained lab techs to operate); and
- Cost-effective (reducing the cost of care).
“This is a platform technology, and it can be used to detect anything from the cancer biomarker OPN or the cardiac stress marker troponin, to the Borrelia spirochete that causes Lyme disease,” Patterson says. “To make different types of sensors, we simply functionalize each channel in the device with a unique capture molecule (like an antibody) that is specific to the target.”
HPV: FROM LDT TO IVD
Trovagene’s urine-based assay for high-risk (HR) human papilloma virus (HPV) offers a novel way to detect the most common sexually transmitted infection in the United States. The company’s proprietary urine-based DNA assay identifies the presence or absence of 13 known HR-HPV genotypes.
Trovagene scientists have identified specific sequences in the HPV E1 gene that discriminate between high-risk and low-risk HPV genotypes. “Targeting the E1 DNA sequence allows for a sensitive molecular assay that can be performed with the convenience, comfort, and flexibility of a urine sample,” says Keith McCormick, Trovagene vice president of sales and sales operations. “However, the assay does not identify which specific high-risk genotype is detected. A positive test result may indicate the need for further testing or follow-up, as determined by a physician or healthcare provider.”
Trovagene’s urine-based HR-HPV assay achieves high sensitivity—nearly as good as established cervical-based HPV tests, says McCormick. “The Trovagene HR-HPV assay is a noninvasive option that may improve the adoption and acceptance rate of HPV testing globally.”
Trovagene’s laboratory is certified under the requirements of the Clinical Laboratory Improvement Amendments of 1988 (CLIA), and is accredited to offer diagnostic services by the College of American Pathologists. McCormick notes that the company’s urine-based HR-HPV assay originated in this setting, and was first made available in 2013 as a laboratory-developed test (LDT).
But today, says McCormick, the company is pursuing a new path for its HR-HPV assay. “The company is in the planning stages for pivotal studies, and is open to pursuing strategic partnership opportunities to commercialize the assay globally,” he says.
The Trovagene HR-HPV assay was the subject of two pilot studies whose results were released during 2014. In the first study, published in the Journal of Clinical Virology, results demonstrated that the company’s assay had a greater than 90% sensitivity for identifying women with high-grade cervical intraepithelial neoplasia (CIN2/3).1 Using patient-matched cervical samples, the study found the performance of the HR-HPV assay to be comparable to that of linear array HPV genotype testing.
In the second study, presented last August at the 2014 International Papillomavirus Conference, in Seattle, standardization of urine collection was examined as a basis for considering urine as a viable clinical specimen for high-risk HPV testing.2 The researchers confirmed that the sensitivity of the test is comparable to tests using physician-collected cervical brush samples, but noted that participants preferred urine collection to brush collection for HR-HPV testing.
HIGH-SPEED TESTING FOR SUPERBUGS
Each year in the United States, at least two million people are infected with bacteria that are resistant to antibiotics. According to the US Centers for Disease Control and Prevention, some 23,000 die as a direct result of such infections.
When “superbugs” like methicillin-resistant Staphylococcus aureus (MRSA) or carbapenem-resistant Enterobacteriaceae (CRE) strike, patients typically endure a delay of 2 to 3 days before current microbiology laboratory methods provide the bacterial identification and antibiotic susceptibility information required to guide treatment. In the meantime—and sometimes even afterward—physicians may administer an array of antibiotics, in the hope that some combination of agents will be effective. The results of this process—delays in appropriate treatment, greater patient distress, poorer outcomes, higher costs, and global diminution of antibiotic effectiveness (as microbes adapt to them)—are unacceptable, says Joen T. Johansen, head of marketing at Accelerate Diagnostics Inc.
An in vitro diagnostics company focused on the challenge of antibiotic-resistant organisms, Accelerate Diagnostics is developing what the company describes as a fully automated instrument for high-speed identification and antibiotic susceptibility testing (ID/AST) of infectious pathogens. Using Accelerate’s automated digital microscopy and image-analysis software, microbiologists can determine the effectiveness of an array of antibiotics in real time, as they act on bacteria from infected patients’ samples, speeding drug selection and optimizing patient treatment, Johansen says.
Accelerate’s scientists adapted standard diagnostic testing principles to take advantage of advances in imaging technology, computing capability, and surface chemistry. They eliminated time-consuming culturing for colony isolation, and created innovative methods to extract, immobilize, and analyze live bacterial cells. Using automated digital microscopy, they have refined a high-resolution bacterial growth analysis technology, according to the company.
“There’s no question that delayed test results are contributing to loss of life and soaring healthcare costs,” says Pete Bantock, chief commercial officer at Accelerate. “The good news is that our technology can address those delays, turning what could take up to 3 days into a matter of hours, and allowing physicians and pharmacists to determine the correct course of action in treating a patient. This type of technology has the capacity to transform the treatment of critically ill patients with antibiotic-resistant infections,” he says.
In February, the National Institutes of Health awarded a 5-year, $5 million grant to Denver Health Medical Center and Accelerate Diagnostics to develop a fast and reliable whole-blood identification and categorical susceptibility test for CRE. The ID/AST system is currently available for research use only, but Accelerate plans to seek FDA clearance of the system and associated tests for positive blood cultures upon successful completion of an upcoming clinical trial.
PAINLESS ALLERGY TESTING
Immunovent is a New York City-based bioventure that is focusing on the commercialization of novel technologies for diagnosing allergies. For its initial product, the company is developing a platform technology called the local antibody mucosal brush diagnostic (LAMB-Dx). The LAMB-Dx test uses a soft brush to painlessly collect cells from the nose and mouth, which allows for accurate detection and diagnosis of diseases, including airborne and food allergies.
In a competition held in December in New York City, Mid-Atlantic Bio Angels (MABA) selected Immunovent as the “best of the best” among previous winners of MABA’s first pitch life science events during 2014. Kate Rochlin, PhD, Immunovent cofounder and chief science officer, said the company anticipates that the recognition that comes with this title may help it continue to develop its LAMB-Dx assay and get it into physicians’ hands.
Immunovent’s LAMB-Dx is a sophisticated and convenient test for allergies. The needle-free test works with minute cell samples that are easily and painlessly collected with a soft brush. A proprietary process is then used to detect specific proteins in the samples that indicate the presence of a food or airborne allergy.
According to Immunovent, allergies affect more than a quarter of the population. However, conventional blood and skin tests are often unable to accurately diagnose patients. For allergy sufferers this is critical, because the allergic reactions that result from exposure to allergens can be halted or reversed using targeted immunotherapies, but only if the allergies are accurately diagnosed. Because the LAMB-Dx method draws samples directly from the mouth and nose, where symptoms are typically present, it is expected to result in a more clinically relevant diagnosis. This novel diagnostic test is also able to diagnose allergy patients whose blood- or skin-based tests are not accurate or are inconsistent with their clinical symptoms.
Initially, Immunovent plans to use the platform to develop a convenient test for peanut allergies, a persistent and potentially dangerous food allergy that affects more than three million Americans.
Immunovent CEO Erick Berglund, PhD, notes that the LAMB-Dx platform has progressed during its current preclinical development phase. Specifically, the company has been working to optimize the release of immunoglobulin E (IgE) antibodies from their cognate receptors on the surface of basophils and mast cells found in patient samples.
“IgE binds to its specific high-affinity IgE receptor (also referred to as the Fc?-R1 receptor) with extremely high affinity (Kd ~10-10 M),” says Berglund. “Immunovent has optimized the disruption of this strong binding—without damaging the IgE—in order to free the IgE antibodies from cell membranes, and thereby make possible a more accurate assessment of IgE in the sample via immunoassay.”
Immunovent recently filed additional intellectual property claims related to its method of IgE release, which Berglund expects will further fortify the company’s global IP portfolio.
According to Berglund, Immunovent intends to bring its initial LAMB-Dx product to market by the end of 2015. The company is now looking for potential development partners, including CLIA-certified laboratories with strong marketing abilities.
SUBCELLULAR IMAGING
Carl Zeiss Microscopy, Thornwood, NY, and the Howard Hughes Medical Institute’s Janelia Research Campus have an exclusive licensing agreement for the commercialization of Bessel beam plane illumination microscopy, also called lattice light sheet microscopy.
The fluorescence imaging method was invented by Nobel prize-winning chemist Eric Betzig, PhD, group leader at the Janelia Research Campus in Ashburn, Va. Bessel beam plane illumination microscopy allows high-speed 3D fluorescence imaging of living cellular and multicellular specimens with nearly isotropic spatial resolution, low photobleaching, and low photodamage. The technology uses special beam conditioning of the light sheet illumination. Enabling very thin illumination beams, it makes light sheet technology usable for applications in cell biology.
The Zeiss-Bessel beam plane technology uses special beam conditioning of the light sheet illumination, making the technology usable for applications in cell biology.
Bessel beam plane illumination microscopy is extending noninvasive imaging, which is already a common feature of the Zeiss light sheet microscope Lightsheet Z.1. The method offers resolution better than existing technologies such as spinning disk. Users of the technology will be able to watch cellular and subcellular division processes in 4D at ultra-high spatial and temporal resolution that has never before been achieved with fluorescence imaging.
Gary Tufel is a contributing writer for CLP. For further information, contact CLP chief editor Steve Halasey via [email protected].
REFERENCES
- Sahasrabuddhe VV, Gravitt PE, Dunn ST, et al. Evaluation of clinical performance of a novel urine-based HPV detection assay among women attending a colposcopy clinic. J Clin Virol. 2014;60(4):414–417; doi: 10.1016/j,jcv.2014.04.016; Epub May 2, 2014.
- Senkomago V, Des Marais A, Rahangdale L, et al. A comparison of urine collection times for the detection of high-risk HPV infection in women. Seattle, Wash: International Papillomavirus Conference, August 2014; available at: www.trovagene.com/sites/default/files/pdfs/publications/posters/Trovagene_Poster_2014_UNC.pdf. Accessed March 20, 2015.