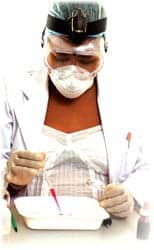
The OncAlert oral cancer product line from Vigilant Biosciences Inc, Fort Lauderdale, Fla, is now available in select markets. The company’s OncAlert oral cancer product line includes the OncAlert oral cancer rapid test and the OncAlert oral cancer lab test, available in countries accepting the CE mark. The tests are based on technology that accurately measures CD44, a tumor-initiating and stem cell-associated biomarker, and total protein levels—markers clinically validated to be associated specifically with oral cancer. The tests are optimized for easy use by collecting samples via an oral saline rinse, and are performed via either a point-of-care lateral-flow assay or a laboratory kit. The test results, along with other clinical factors, can aid in the early diagnosis of oral cancer, potentially even before visual or physical indicators. According to a company spokesperson, Vigilant Biosciences has plans to pursue FDA approval on the OncAlert oral cancer product line in the near future. For more information, visit Vigilant Biosciences.
Oral Cancer Tests Capture Biomarkers Using Saline Rinse