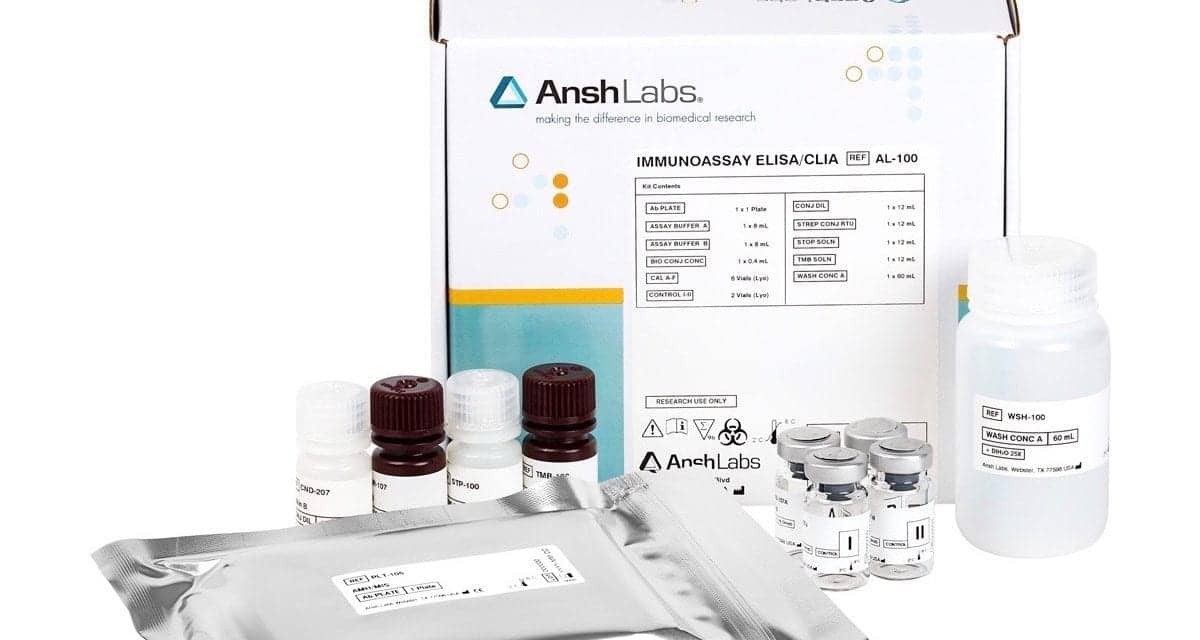
FDA has recently granted de novo market clearance to Ansh Labs, Webster, Tex, for the company’s anti-Müllerian hormone (AMH) assay, picoAMH ELISA, as an aid in the determination of a patient’s menopausal status. The test will be sold in a kit format under the trademark MenoCheck.
Menopause refers to the time in a woman’s life when she stops having a menstrual period and is no longer fertile. During the menopausal transition, the body’s production of estrogen and progesterone, two hormones made by the ovaries, varies greatly. Bones become less dense, making women more vulnerable to fractures. During this period, lipid profiles may undergo change, including increases in low-density lipoprotein (LDL), a type of cholesterol. After menopause, women enter postmenopause, when they are more vulnerable to heart disease and osteoporosis. It is important for women to understand their stage of menopausal transition to learn what additional health risks they may face and any preventive health steps they should take.
Blood levels of AMH have been shown to be highly correlated with the number of primordial follicles in an ovary (that is, a woman’s true ovarian reserve). The number of follicles declines with age, and the natural cause of menopause is the absence of these follicles. The MenoCheck assay was developed to achieve extremely sensitive measurements of AMH, providing a significant new parameter to aid physicians in determining the status of women during menopausal transition.
During menopausal transition, the duration and intensity of women’s physiological changes or vasomotor symptoms is highly variable. Determining where a woman is in this process is clinically important. Presently, menopause is determined retrospectively, since it is clinically defined by the absence of a menstrual cycle (ie, 12 months of amenorrhea).
The picoAMH ELISA is intended for use as an aid in the determination of menopausal status among women aged 42 to 62 years. The assay is the first FDA-cleared AMH test with the sensitivity to quantify declining AMH concentrations among women who are entering their menopausal transition.
The PicoAMH ELISA measures the amount of anti-Müllerian hormone (AMH) in the blood. AMH levels represent one indicator available to clinicians to determine whether a woman is approaching or is likely to have reached her final menstrual period. The PicoAMH ELISA is meant to be used only in conjunction with other clinical assessments and laboratory findings.
“Diagnostic results about a woman’s menopausal status may prompt discussions about preventive care for women experiencing menopausal symptoms,” says Courtney Lias, PhD, director of the division of chemistry and toxicology devices at FDA’s Center for Devices and Radiological Health. “This test, when used in conjunction with other clinical assessments and laboratory findings, can help inform discussions about preventive care, such as ways to help prevent loss in bone mineral density or to address cardiovascular disease, both of which are known to increase after menopause.”
FDA reviewed data submitted by the sponsor that included 690 women, aged 42 to 62, who participated in the multicenter, longitudinal Study of Women’s Health Across the Nation.1 Study data showed that the PicoAMH ELISA performed reasonably well at determining levels of AMH in the blood, identifying women who were more than 5 years away from their final menstrual period, and identifying women who had had their final menstrual period.
A sensitive AMH test result will help physicians to understand the symptoms the patient is experiencing and to select an appropriate treatment to ameliorate those symptoms and prevent complications associated with the onset of menopause. Determining the onset of menopause at an early age, for instance, may indicate to the physician a need to investigate the potential for accelerated bone loss leading to osteoporosis. This is a significant health issue among postmenopausal women.
The new picoAMH ELISA device uses well-established chemical principles, methodology, and components for the quantitative determination of AMH in human serum. The specificity of the antibodies, recombinant human AMH used for calibrators, and enhanced sensitivity of the picoAMH ELISA are advantageous for the intended use of the device as an aid in the determination of menopausal status.
FDA advises that clinicians should carefully evaluate PicoAMH ELISA results in the context of a full clinical work-up to ensure that contraceptives are not discontinued in women who have not yet reached menopause and that uterine bleeding due to endometrial cancer is not dismissed as a diagnosis. The PicoAMH ELISA should not be used to assess a woman’s fertility status or to monitor or predict the ovarian response in women undergoing or planning to undergo fertility treatments.
FDA reviewed data for the PicoAMH ELISA through the de novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type. The agency also established special controls that set forth the agency’s expectations for ensuring the accuracy, clinical performance, and labeling of tests intended to be used as an aid in the determination of a patient’s menopausal status.
For further information, visit Ansh Labs.
Reference
- Study of Women’s Health Across the Nation [online]. Bethesda, Md: SWAN Study, 2018. Available at: www.swanstudy.org. Accessed December 11, 2018.
Featured image: The MenoCheck picoAMH ELISA kit by Ansh Labs.