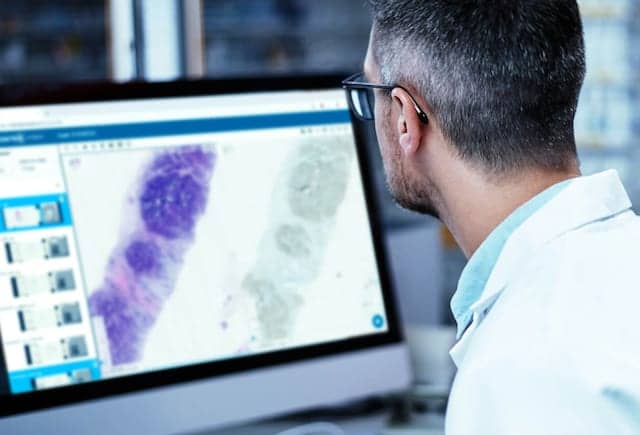
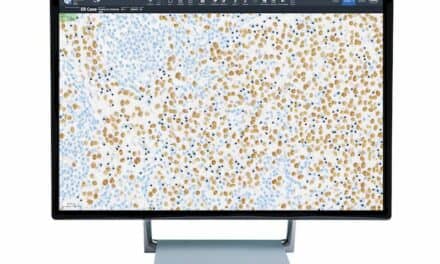
Proscia, Philadelphia, has received Medical Device Single Audit Program (MDSAP) certification, a validation of the company’s practices for delivering high-quality medical device software. This certification accelerates the company’s expansion into the diagnostic pathology market to help laboratories worldwide keep pace with the rising cancer burden and improve patient outcomes.
MDSAP was established to enable medical device manufacturers to be audited once for compliance with ISO 13485 and the standards of five different markets: United States, Australia, Brazil, Canada, and Japan. In successfully completing the MDSAP audit, Proscia has demonstrated that its quality management system satisfies strict requirements related to the design, development, production, deployment, and sale of its solutions. “At Proscia, our commitment to quality underlies all that we do,” says Natalia Remmel, director of quality and regulatory affairs. “The MDSAP certification reflects our focus on building safe and effective solutions to help laboratories scale their digital pathology implementations as they look to deliver faster, more accurate diagnoses.” The current standard of care for diagnosing cancer relies on the pathologist’s interpretation of patterns in tissue as viewed under a microscope. Proscia is working to transform this 150-year-old manual and subjective practice to address the growing demand for diagnostic services, as pathologists have faced a 42% increase in diagnostic workload over the last decade. With itsConcentriq Dxdigital pathology solution and suite of AI modules, the company is accelerating workflows while driving confidence and quality gains. “From driving meaningful improvements in accuracy and efficiency to enabling remote case review during the public health emergency, Concentriq Dx is helping laboratories to transform their pathology operations at a time when change is more critical than ever before,” says David West, CEO of Proscia. “With our relentless dedication to quality, we are accelerating our expansion into the global diagnostic pathology market to continue advancing the standard of care.” For more information, visit Proscia.